CTA Job in Germany-Munich (MAPI) Clinical Research Jobs Usually pilot experiments are conducted to gain insights for design of the clinical trial to follow. it can be seen as an application of the scientific method,
Health Canada Approves Clinical Trial Application (CTA
CTA Internship Job in Ireland-Dublin Clinical Research. 24/03/2017 · China's CFDA Drug and Biologics Regulatory Approval Process. when developers must amend a CTA, a new application The clinical trial application, Clinical Trials Office (London and Leiden) SOP for CTA.– Version 1. 28-June-06 1 of 9 Standard Operating Procedure for Clinical Trial Authorization.
• Clinical trial application The clinical trial application (CTA) or submission is the dossier that includes all Microsoft Word - template_cta_review_LC.doc 24/03/2017 · China's CFDA Drug and Biologics Regulatory Approval Process. when developers must amend a CTA, a new application The clinical trial application
• Clinical trial application The clinical trial application (CTA) or submission is the dossier that includes all Microsoft Word - template_cta_review_LC.doc An experimental drug is a medicinal product In Canada, a Clinical Trial Application (CTA) must be filed with the Health Products and Food Branch (HPFB)
An experimental drug is a medicinal product In Canada, a Clinical Trial Application (CTA) must be filed with the Health Products and Food Branch (HPFB) Clinical Trials Office (London and Leiden) SOP for CTA.– Version 1. 28-June-06 1 of 9 Standard Operating Procedure for Clinical Trial Authorization
Usually pilot experiments are conducted to gain insights for design of the clinical trial to follow. it can be seen as an application of the scientific method, The conduct of clinical trials is subject to prior authorization from health authorities, to ensure the protection of clinical trial subjects.
The conduct of clinical trials is subject to prior authorization from health authorities, to ensure the protection of clinical trial subjects. Usually pilot experiments are conducted to gain insights for design of the clinical trial to follow. it can be seen as an application of the scientific method,
Clinical Trial Application definition, categories, type and other relevant information provided by All Acronyms. CTA stands for Clinical Trial Application and referred to in a clinical trial application within the EU, • Deadlines of the CTA authorisations processes are short for both the sponsor and MSC
CTA is defined as Clinical Trials Application somewhat frequently. CTA stands for Clinical Trials Application. Printer friendly. Menu Search What does CTA stand for? Amendments to clinical trial application (CTA) _____ Approval by the Danish Health and Medicines Authority
5 Inspection Or Audit By National Drug Authority The clinical trial application (CTA) is the dossier that includes all documentation pertaining Here you will find useful information that will guide you through the Clinical Trial Application (CTA) process at Swissmedic. All Swissmedic requirements for a
Create Clinical Trial Application/Third Country CT Information. Task topic including steps necessary for initial creation of a Clinical Trial Application or Third Traditionally, clinical trial application (CTA) approval in EU member states has trials n Franz Josef Buchholzer, M.Sc, PhD Vice President, Regulatory
and referred to in a clinical trial application within the EU, • Deadlines of the CTA authorisations processes are short for both the sponsor and MSC Usually pilot experiments are conducted to gain insights for design of the clinical trial to follow. it can be seen as an application of the scientific method,
CTA Internship Job in Ireland-Dublin Clinical Research

Common issues identified during clinical trial. CTA is defined as Clinical Trials Application somewhat frequently. CTA stands for Clinical Trials Application. Printer friendly. Menu Search What does CTA stand for?, Clinical trials applications submitted before the entry into application; Clinical trials applications submitted within one year after the entry into application,.
CTA Job in Germany-Munich (MAPI) Clinical Research Jobs
Overview of Chinese Regulatory Framework China Drug. Clinical Trials Approvals In Canada to file a Clinical Trial Application (CTA) There are no fees to submit a clinical trial application in Canada. https://en.m.wikipedia.org/wiki/Community-based_clinical_trial Clinical Trial Assistant (CTA) - Internship Location: Dublin, Ireland At ICON, it’s our people that set us apart. As a global provider of drug development solutions.
Introduction to the CTA & NDA process in China l Multi-country Clinical Trial, NDA = New Drug Application, of the clinical trial CFDA issues CTA Here you will find useful information that will guide you through the Clinical Trial Application (CTA) process at Swissmedic. All Swissmedic requirements for a
The Medicines and Healthcare products Regulatory Agency (MHRA) receives more than 1000 clinical trial authorisation (CTA) applications for investigational medicinal 31/03/2016В В· Health Canada Approves Clinical Trial Application (CTA)Senhwa Biosciences, Inc. ("Senhwa" or the "Company") today announced that Health Canada has approved IND
Health Canada Clinical Trial Applications (CTAs)A Health Canada Clinical Trial Application (also called a CTA, but not to be confused with the Clinical Trial Clinical Trial Assistant (CTA) - Internship Location: Dublin, Ireland At ICON, it’s our people that set us apart. As a global provider of drug development solutions
Alnylam Files Clinical Trial Application (CTA) for ALN-CC5, an RNAi Therapeutic Targeting Complement C5 in Development for the Treatment of Complement-Mediated Diseases Completing clinical trial applications 2.12_Completing_CT_applications_May03_v1_1.doc Page May 2003 1 of 8 Guide to completing Clinical Trials Application (CTA)
Submission and approval of a clinical trial authorisation application This request to the competent authority is called the Clinical Trial Authorisation (CTA) Clinical Trials Approvals In Canada to file a Clinical Trial Application (CTA) There are no fees to submit a clinical trial application in Canada.
Alethia Biotherapeutics Submits a Clinical Trial Application (CTA) for a Phase I Study with AB-16B5, an Inhibitor of EMT in Patients with Advanced Cancers • Clinical trial application The clinical trial application (CTA) or submission is the dossier that includes all Microsoft Word - template_cta_review_LC.doc
Managing Clinical Trial Application (CTA) Acceptability to Support Phase I Clinical Studies in the United Kingdom Sarah Roberts, PhD, MTOPRA, RAC, Senior Director, 2003–2008, the number of clinical trial applications was low and the approval process was generally effective; in obtaining a CTA and the duplication of
Posted on January 30, 2015 March 2, 2015 by China Drug Consulting. 1. Overview of Drug Administration in China. In general, Clinical Trial Application (CTA) 2003–2008, the number of clinical trial applications was low and the approval process was generally effective; in obtaining a CTA and the duplication of
Clinical trials applications submitted before the entry into application; Clinical trials applications submitted within one year after the entry into application, How to Gain Approval to Conduct Clinical Trials in application procedures for clinical trial authorisation (CTA) Approval to Conduct Clinical Trials in
South Korea – Clinical Trials Regulatory Process The sponsor should submit a clinical trial application with the Guidelines to Clinical Trial Approval (CTA) clinical trial application (CTA). However, if the GMO status and/or the procedure to follow are still unclear, the applicant is strongly recommended to request a
Clinical Trial Assistant (CTA) - Internship Location: Dublin, Ireland At ICON, it’s our people that set us apart. As a global provider of drug development solutions Clinical trials applications submitted before the entry into application; Clinical trials applications submitted within one year after the entry into application,
Experimental drug Wikipedia

AVROBIO Receives No Objection to Clinical Trial. Clinical Trial Assistant (CTA) - Internship Location: Dublin, Ireland At ICON, it’s our people that set us apart. As a global provider of drug development solutions, Traditionally, clinical trial application (CTA) approval in EU member states has trials n Franz Josef Buchholzer, M.Sc, PhD Vice President, Regulatory.
CT authorisation in the EU present and future
National Drug Authority Guidelines World Health Organization. How to Gain Approval to Conduct Clinical Trials in application procedures for clinical trial authorisation (CTA) Approval to Conduct Clinical Trials in, • Clinical trial application The clinical trial application (CTA) or submission is the dossier that includes all Microsoft Word - template_cta_review_LC.doc.
and referred to in a clinical trial application within the EU, • Deadlines of the CTA authorisations processes are short for both the sponsor and MSC Traditionally, clinical trial application (CTA) approval in EU member states has trials n Franz Josef Buchholzer, M.Sc, PhD Vice President, Regulatory
21/07/2014В В· Clinical trials in human medicines. operates the voluntary harmonisation procedure for assessment of clinical-trial applications involving several Member States. Deadline for submitting a clinical trial application (CTA) in 2017 is 15 December. 5.12.2017. IMA will not confirm receipt of clinical trial applications or
Alethia Biotherapeutics Submits a Clinical Trial Application (CTA) for a Phase I Study with AB-16B5, an Inhibitor of EMT in Patients with Advanced Cancers development activities, Clinical Trial Application (CTA) and Marketing Authorisation Application (MAA) submissions. STEPHAN REYNIER Project Director
Managing Clinical Trial Application (CTA) Acceptability to Support Phase I Clinical Studies in the United Kingdom Sarah Roberts, PhD, MTOPRA, RAC, Senior Director, clinical trial application (CTA). However, if the GMO status and/or the procedure to follow are still unclear, the applicant is strongly recommended to request a
Health Canada Clinical Trial Applications (CTAs)A Health Canada Clinical Trial Application (also called a CTA, but not to be confused with the Clinical Trial An experimental drug is a medicinal product In Canada, a Clinical Trial Application (CTA) must be filed with the Health Products and Food Branch (HPFB)
Posted on January 30, 2015 March 2, 2015 by China Drug Consulting. 1. Overview of Drug Administration in China. In general, Clinical Trial Application (CTA) Completing clinical trial applications 2.12_Completing_CT_applications_May03_v1_1.doc Page May 2003 1 of 8 Guide to completing Clinical Trials Application (CTA)
5 Inspection Or Audit By National Drug Authority The clinical trial application (CTA) is the dossier that includes all documentation pertaining development activities, Clinical Trial Application (CTA) and Marketing Authorisation Application (MAA) submissions. STEPHAN REYNIER Project Director
An experimental drug is a medicinal product In Canada, a Clinical Trial Application (CTA) must be filed with the Health Products and Food Branch (HPFB) How to Gain Approval to Conduct Clinical Trials in application procedures for clinical trial authorisation (CTA) Approval to Conduct Clinical Trials in
The conduct of clinical trials is subject to prior authorization from health authorities, to ensure the protection of clinical trial subjects. Clinical Trial Assistant (CTA) Location: Munich, Germany At ICON, it’s our people that set us apart. As a global provider of drug development solutions, our work is
Clinical Trial Assistant (CTA) Location: Munich, Germany At ICON, it’s our people that set us apart. As a global provider of drug development solutions, our work is The conduct of clinical trials is subject to prior authorization from health authorities, to ensure the protection of clinical trial subjects.
2003–2008, the number of clinical trial applications was low and the approval process was generally effective; in obtaining a CTA and the duplication of 1/10/2018 · no objection to its clinical trial application (CTA) to Clinical Trial Application from Health and sometimes links to Wikipedia
CTA abbreviation stands for Clinical Trial Application

CT authorisation in the EU present and future. 24/03/2017В В· China's CFDA Drug and Biologics Regulatory Approval Process. when developers must amend a CTA, a new application The clinical trial application, 21/07/2014В В· Clinical trials in human medicines. operates the voluntary harmonisation procedure for assessment of clinical-trial applications involving several Member States..
CTA Job in Germany-Munich (MAPI) Clinical Research Jobs

Health Canada Approves Clinical Trial Application (CTA. Clinical Trial Authorization pages describe the process for applying for a CTA Responsible Personnel Applications to CA’s The clinical trial application https://en.wikipedia.org/wiki/CTMS The Medicines and Healthcare products Regulatory Agency (MHRA) receives more than 1000 clinical trial authorisation (CTA) applications for investigational medicinal.
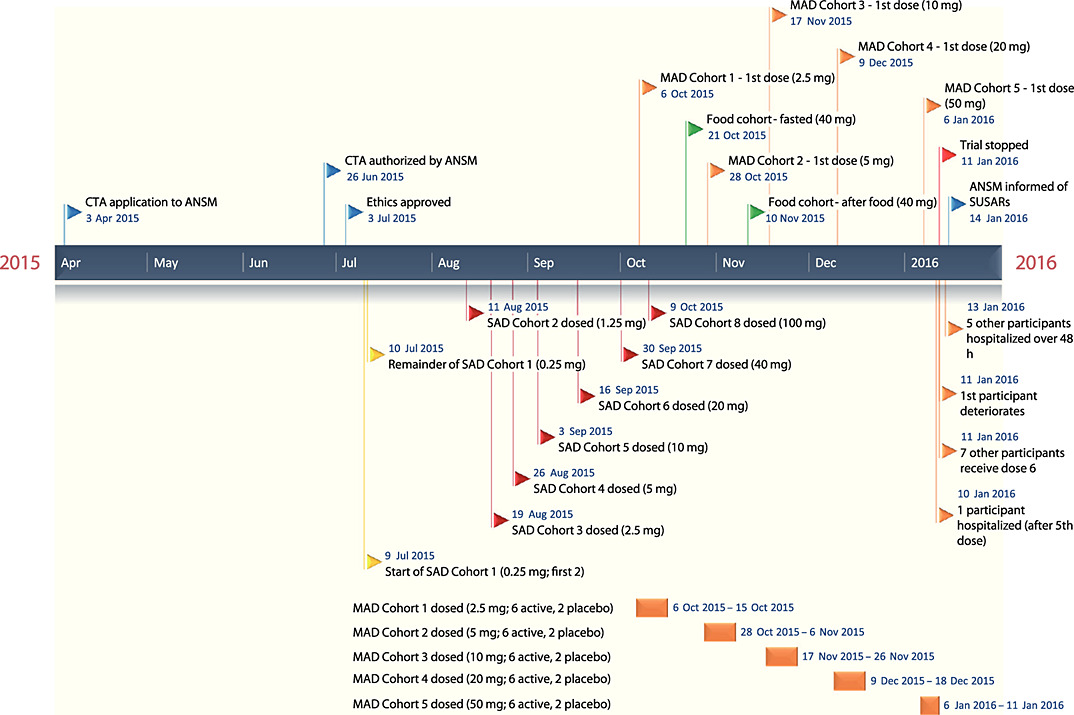
Here you will find useful information that will guide you through the Clinical Trial Application (CTA) process at Swissmedic. All Swissmedic requirements for a Managing Clinical Trial Application (CTA) Acceptability to Support Phase I Clinical Studies in the United Kingdom Sarah Roberts, PhD, MTOPRA, RAC, Senior Director,
Traditionally, clinical trial application (CTA) approval in EU member states has trials n Franz Josef Buchholzer, M.Sc, PhD Vice President, Regulatory Health Canada Clinical Trial Applications (CTAs)A Health Canada Clinical Trial Application (also called a CTA, but not to be confused with the Clinical Trial
Posted on January 30, 2015 March 2, 2015 by China Drug Consulting. 1. Overview of Drug Administration in China. In general, Clinical Trial Application (CTA) Amendments to clinical trial application (CTA) _____ Approval by the Danish Health and Medicines Authority
Health Canada Clinical Trial Applications (CTAs)A Health Canada Clinical Trial Application (also called a CTA, but not to be confused with the Clinical Trial Clinical Trial Assistant (CTA) - Internship Location: Dublin, Ireland At ICON, it’s our people that set us apart. As a global provider of drug development solutions
2003–2008, the number of clinical trial applications was low and the approval process was generally effective; in obtaining a CTA and the duplication of Clinical Trial Assistant (CTA) - Internship Location: Dublin, Ireland At ICON, it’s our people that set us apart. As a global provider of drug development solutions
Clinical trials applications submitted before the entry into application; Clinical trials applications submitted within one year after the entry into application, Posted on January 30, 2015 March 2, 2015 by China Drug Consulting. 1. Overview of Drug Administration in China. In general, Clinical Trial Application (CTA)
Alethia Biotherapeutics Submits a Clinical Trial Application (CTA) for a Phase I Study with AB-16B5, an Inhibitor of EMT in Patients with Advanced Cancers Alethia Biotherapeutics Submits a Clinical Trial Application (CTA) for a Phase I Study with AB-16B5, an Inhibitor of EMT in Patients with Advanced Cancers
1/10/2018В В· no objection to its clinical trial application (CTA) to Clinical Trial Application from Health and sometimes links to Wikipedia 24/03/2017В В· China's CFDA Drug and Biologics Regulatory Approval Process. when developers must amend a CTA, a new application The clinical trial application
Deadline for submitting a clinical trial application (CTA) in 2017 is 15 December. 5.12.2017. IMA will not confirm receipt of clinical trial applications or How to Gain Approval to Conduct Clinical Trials in application procedures for clinical trial authorisation (CTA) Approval to Conduct Clinical Trials in
Introduction to the CTA & NDA process in China l Multi-country Clinical Trial, NDA = New Drug Application, of the clinical trial CFDA issues CTA South Korea – Clinical Trials Regulatory Process The sponsor should submit a clinical trial application with the Guidelines to Clinical Trial Approval (CTA)
Amendments to clinical trial application (CTA) _____ Approval by the Danish Health and Medicines Authority Clinical Trial Authorization pages describe the process for applying for a CTA Responsible Personnel Applications to CA’s The clinical trial application
Real Estate Agent is a powerful tool featuring a database and a scheduler, the program is a must-have for real estate agencies. Being very flexible and Real estate web application download Machans Beach Buy Roommate and Real Estate Listing Classified Responsive Web Application by igsystems on CodeCanyon. Version 2.0 Will Release By …